When yellowish patches appear around the eyelids, most people immediately assume high cholesterol is to blame. However, many individuals discover they have xanthelasma with normal cholesterol levels, creating a confusing medical paradox that challenges conventional understanding. These soft, cholesterol-filled deposits can develop even when lipid panels show perfectly healthy numbers, leaving patients wondering what's really happening beneath their skin.
The presence of xanthelasma with normal cholesterol affects approximately 0.5-1.5% of the general population, with rates increasing significantly after age 40[1]. While these yellowish plaques are often associated with cardiovascular risk and lipid disorders, research shows that 40-50% of people with xanthelasma have completely normal cholesterol levels[2]. This surprising statistic highlights the complexity of lipid metabolism and the need for deeper understanding beyond simple cholesterol testing.
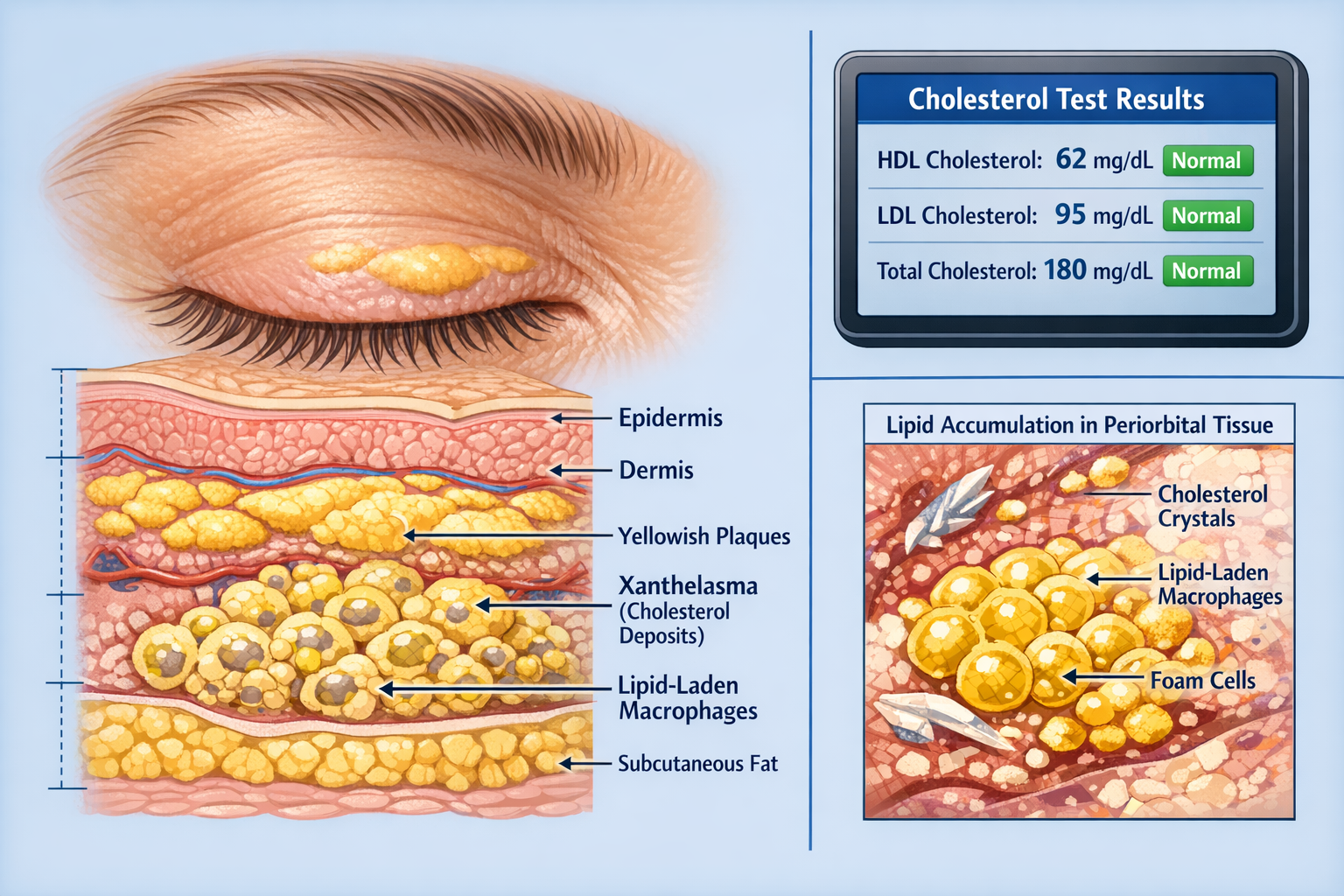
Xanthelasma palpebrarum represents the most common form of cutaneous xanthoma, appearing as soft, yellowish plaques typically located on the medial aspects of the upper and lower eyelids[3]. These deposits consist of lipid-laden macrophages, also called foam cells, that accumulate in the dermis layer of the skin. The term derives from the Greek words "xanthos" (yellow) and "elasma" (plate), perfectly describing their characteristic appearance.
The deposits usually start small, often just a few millimeters in diameter, but can gradually enlarge over months or years. They're typically:
Unlike other types of skin lesions, xanthelasma deposits don't cause functional problems with vision or eyelid movement, but they can significantly impact cosmetic appearance and self-confidence.
Traditional medical understanding links xanthelasma directly to hyperlipidemia—elevated levels of fats in the blood. However, the reality proves far more nuanced. When examining xanthelasma with normal cholesterol, researchers have identified several mechanisms that explain this apparent contradiction:
Local Lipid Metabolism Abnormalities: The skin around the eyes may process lipids differently than other body tissues. Even with normal circulating cholesterol, local cellular mechanisms can lead to lipid accumulation in periorbital tissue[4].
Apolipoprotein Irregularities: Standard cholesterol tests measure total cholesterol, LDL, HDL, and triglycerides, but they don't capture the full picture of lipid transport proteins. Abnormalities in apolipoprotein A1, B, and E can cause xanthelasma formation despite normal cholesterol numbers[5].
HDL Dysfunction: Having "normal" HDL cholesterol levels doesn't guarantee proper HDL function. Dysfunctional HDL particles may fail to remove cholesterol efficiently from tissues, leading to localized deposits[6].
Genetics plays a substantial role in xanthelasma development, particularly in cases where cholesterol levels remain normal. Several inherited conditions increase susceptibility:
Familial Hypercholesterolemia (FH): While typically associated with elevated cholesterol, some FH variants present with normal lipid panels but altered lipid particle composition[7]. Family history of xanthelasma, even without documented cholesterol problems, increases individual risk by 3-5 times.
Apolipoprotein E Polymorphisms: The APOE gene comes in several variants (E2, E3, E4), and certain combinations affect how the body processes fats. The E2/E2 genotype, found in approximately 1% of the population, can lead to xanthelasma formation with normal or even low LDL cholesterol[8].
Ethnic Variations: Studies show higher prevalence rates in certain populations:
Population GroupXanthelasma PrevalenceNormal Cholesterol CasesAsian descent1.5-3.0%45-55%Mediterranean1.0-2.5%40-50%Northern European0.5-1.5%35-45%African descent0.3-1.0%30-40%
The relationship between age and xanthelasma development extends beyond simple time-based accumulation. Xanthelasma with normal cholesterol becomes increasingly common after age 40, with peak incidence between 50-60 years[9].
Menopausal Changes: Women experience significant shifts in lipid metabolism during and after menopause. Declining estrogen levels affect HDL function and cholesterol transport, potentially triggering xanthelasma formation even when cholesterol numbers remain within normal ranges[10]. This explains why women develop xanthelasma more frequently than men, with a female-to-male ratio of approximately 1.5:1.
Thyroid Function: Hypothyroidism, even subclinical cases with minimal symptoms, can alter lipid metabolism in ways that standard cholesterol tests don't detect. The thyroid gland regulates LDL receptor activity, and reduced thyroid function can impair cholesterol clearance at the cellular level while maintaining seemingly normal blood lipid levels[11].
Beyond cholesterol, several metabolic conditions contribute to xanthelasma development:
Metabolic Syndrome Components:
Even with normal cholesterol, these factors create an inflammatory environment that promotes lipid deposition in tissues[12]. The presence of metabolic syndrome increases xanthelasma risk by approximately 2.5-fold, independent of cholesterol levels.
Liver Function Abnormalities: The liver orchestrates cholesterol synthesis, metabolism, and excretion. Conditions like primary biliary cholangitis, non-alcoholic fatty liver disease (NAFLD), or subtle liver enzyme elevations can disrupt lipid processing without necessarily raising blood cholesterol[13].
Chronic Kidney Disease: Renal dysfunction alters lipid profiles in complex ways. Patients with chronic kidney disease often develop dyslipidemia characterized by normal or low LDL but significantly altered HDL composition and function[14].
Experienced healthcare providers can typically diagnose xanthelasma through visual examination alone. The characteristic appearance—soft, yellowish plaques on the medial eyelids—is distinctive enough to differentiate from other periorbital conditions.
However, several conditions can mimic xanthelasma:
Professional evaluation at a specialized skin clinic ensures accurate diagnosis and appropriate treatment planning. Dermatoscopy, a non-invasive imaging technique, can help confirm the diagnosis by revealing the characteristic yellow-orange color and homogeneous structure of lipid deposits.
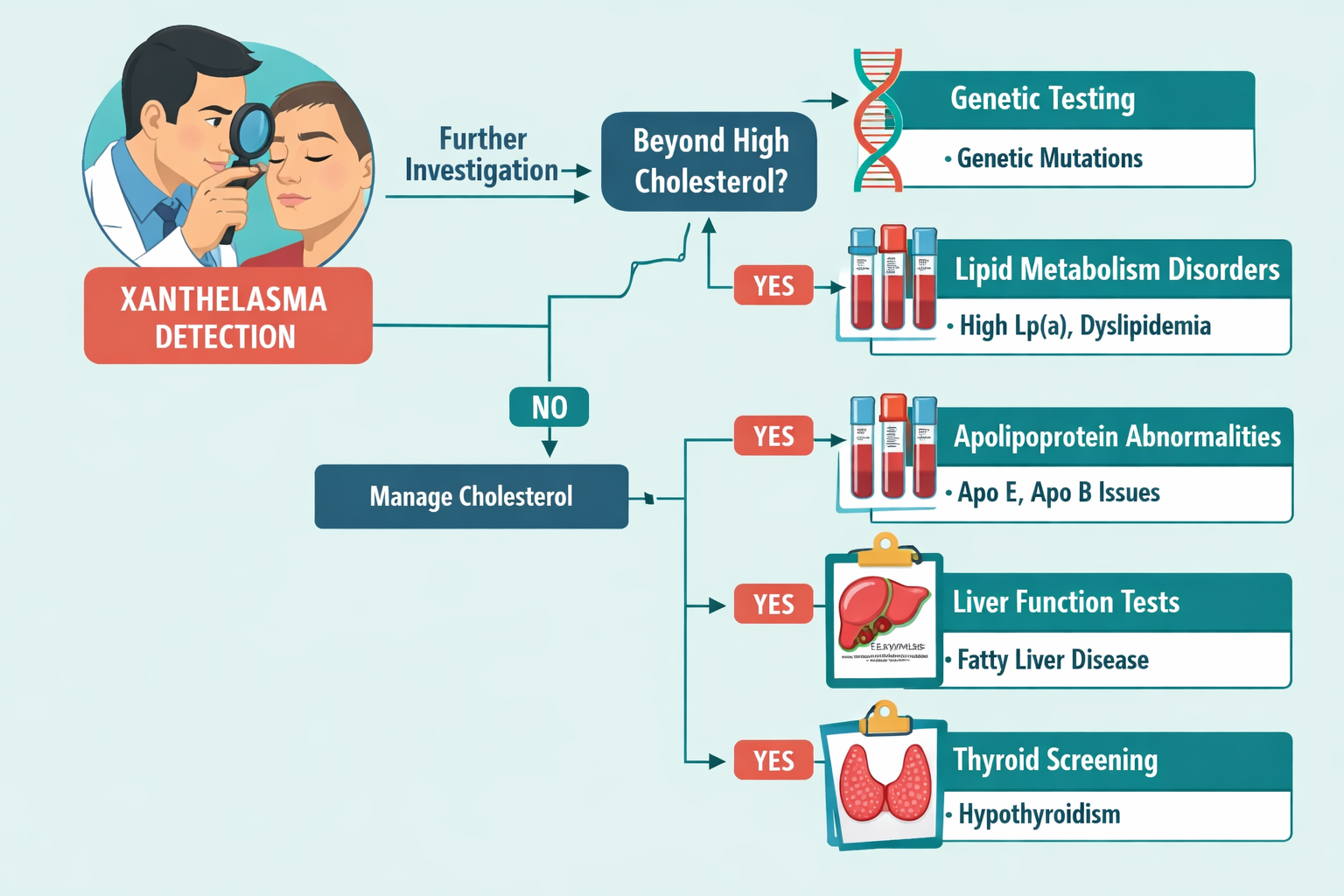
When xanthelasma with normal cholesterol presents, standard lipid panels prove insufficient. Advanced testing provides crucial insights:
Advanced Lipid Panel Components:
Recommended Testing Protocol:
✓ Fasting lipid panel (baseline)
✓ Apolipoprotein B and A1
✓ Lipoprotein(a)
✓ Advanced lipoprotein particle analysis (NMR or ion mobility)
✓ Thyroid function tests (TSH, Free T4)
✓ Liver function tests (AST, ALT, GGT)
✓ Fasting glucose and HbA1c
✓ High-sensitivity C-reactive protein (inflammation marker)
For patients with xanthelasma with normal cholesterol, particularly those with family history or early onset (before age 40), genetic testing can identify underlying inherited lipid disorders:
Candidate Genes for Testing:
Genetic counseling helps interpret results and assess familial risk. Identifying a genetic mutation can guide treatment decisions and prompt screening of family members.
Skin Biopsy Considerations: While rarely necessary for diagnosis, skin biopsy definitively confirms xanthelasma by revealing foam cells and lipid deposits in the dermis. Biopsy becomes relevant when:
One of the most important clinical questions surrounding xanthelasma with normal cholesterol concerns cardiovascular risk. Does the presence of these deposits signal increased heart disease risk even when cholesterol levels appear healthy?
Research provides a nuanced answer: Yes, but the relationship is complex[17].
Key Research Findings:
A landmark Danish study following 12,745 participants for 33 years found that individuals with xanthelasma had:
Importantly, these associations persisted even after adjusting for cholesterol levels and other cardiovascular risk factors[18].
"Xanthelasma appears to be an independent marker of cardiovascular risk, reflecting subtle lipid metabolism abnormalities that standard cholesterol testing doesn't capture." — Journal of the American College of Cardiology
Several mechanisms explain why xanthelasma with normal cholesterol still signals cardiovascular risk:
Subclinical Dyslipidemia: Advanced lipid testing often reveals abnormalities invisible to standard panels—elevated ApoB, high Lp(a), dysfunctional HDL, or increased small dense LDL particles[19].
Chronic Inflammation: Xanthelasma formation involves inflammatory processes. The same inflammatory pathways contribute to atherosclerosis development, creating parallel but related disease processes[20].
Endothelial Dysfunction: The presence of xanthelasma may indicate impaired vascular endothelial function, a critical early step in cardiovascular disease development that occurs independently of cholesterol levels[21].
Shared Genetic Factors: Genetic variants affecting lipid metabolism may simultaneously increase susceptibility to both xanthelasma and cardiovascular disease through overlapping but distinct pathways.
Patients with xanthelasma with normal cholesterol require individualized cardiovascular risk assessment:
Recommended Cardiovascular Evaluation:
📋 Clinical Assessment:
📋 Laboratory Testing:
📋 Imaging Studies (when indicated):
Risk Calculator Limitations: Standard cardiovascular risk calculators (Framingham, ASCVD) may underestimate risk in xanthelasma patients with normal cholesterol. Consider these tools as baseline assessments requiring clinical judgment and additional testing.
Even with normal cholesterol levels, addressing modifiable risk factors and optimizing lipid metabolism can prevent xanthelasma progression and potentially reduce lesion size:
Dietary Interventions:
While dietary cholesterol has limited impact on blood cholesterol for most people, specific dietary patterns may influence local lipid metabolism:
Physical Activity: Regular exercise improves HDL function, reduces inflammation, and enhances overall lipid metabolism. Aim for:
Weight Management: For patients with metabolic syndrome components, even modest weight loss (5-10% of body weight) can significantly improve lipid metabolism[22].
Statins: The role of statins in xanthelasma with normal cholesterol remains controversial. While statins don't reliably reduce existing xanthelasma, they may:
Statin therapy should be considered when:
Fibrates: These medications primarily lower triglycerides and can raise HDL. They may benefit patients with:
PCSK9 Inhibitors: These newer injectable medications dramatically lower LDL and ApoB. While expensive, they may be appropriate for:
Topical Treatments: Various topical agents have been tried with limited success:
Success rates with topical treatments remain modest (20-40%), with frequent recurrence[23].
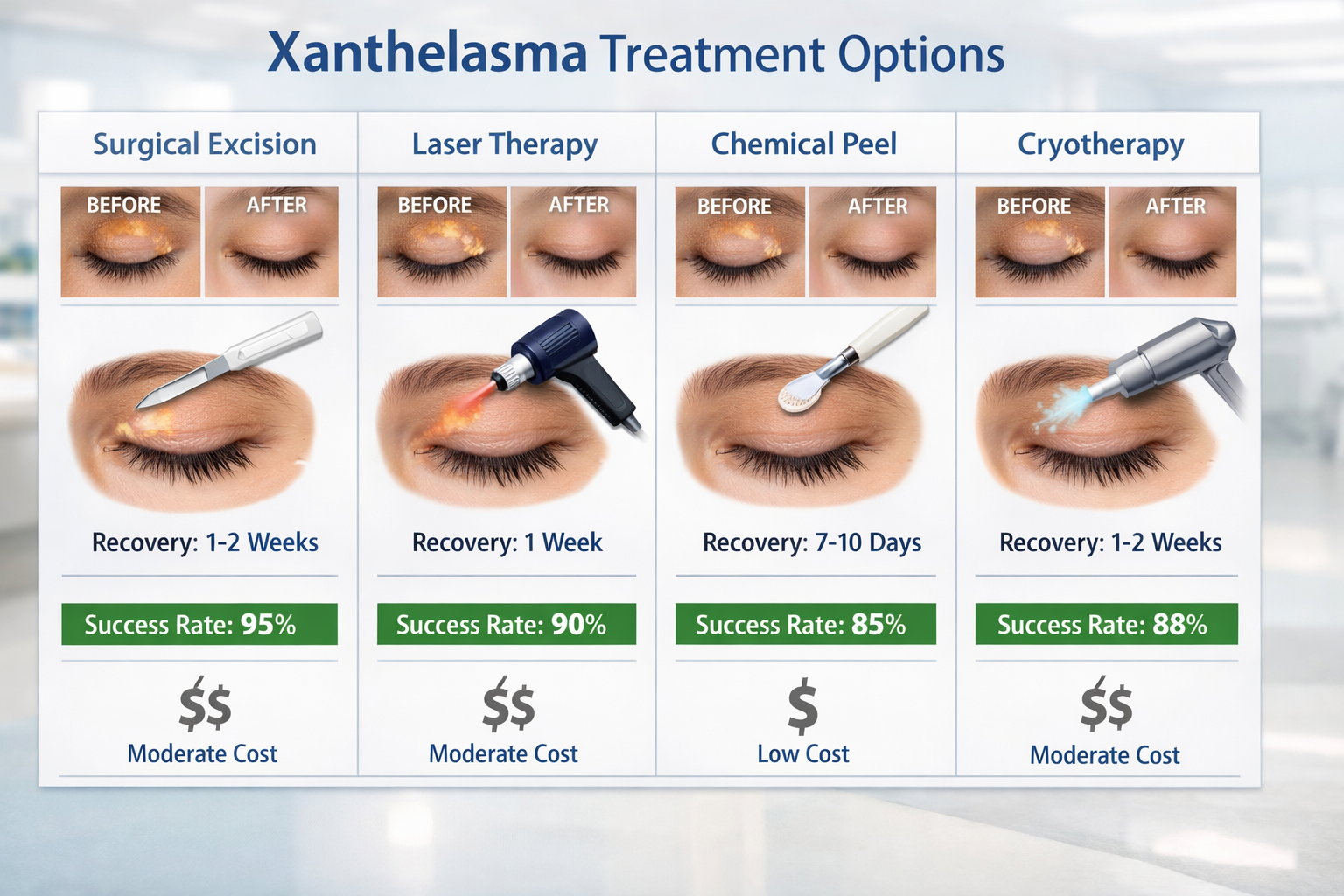
For many patients, the primary concern with xanthelasma is cosmetic. Several removal techniques offer varying success rates and recurrence risks:
Surgical Excision:
The gold standard for xanthelasma removal involves careful surgical excision under local anesthesia. This approach offers:
✅ Advantages:
❌ Disadvantages:
Professional surgical removal at facilities like The Minor Surgery Center ensures optimal cosmetic outcomes with minimal scarring.
Laser Therapy:
Several laser types effectively treat xanthelasma:
CO2 Laser: Vaporizes tissue layer by layer with precision
Erbium:YAG Laser: Gentler than CO2, less thermal damage
Pulsed Dye Laser: Targets blood vessels, less effective for lipid deposits
Chemical Peels:
Trichloroacetic acid (TCA) peels at concentrations of 35-50% can effectively remove superficial xanthelasma:
Cryotherapy:
Freezing with liquid nitrogen destroys lipid-laden cells:
Radiofrequency Ablation:
Newer technique using controlled heat:
Treatment selection for xanthelasma with normal cholesterol depends on multiple factors:
Lesion Characteristics:
Patient Factors:
Treatment Comparison Table:
MethodSuccess RateRecurrence RiskScarring RiskRecovery TimeCostSurgical Excision90-95%Low (10-20%)Moderate7-14 days$$$CO2 Laser70-85%Moderate (20-40%)Moderate7-10 days$$$Erbium Laser65-80%Moderate-High (25-45%)Low-Moderate5-7 days$$$Chemical Peel60-75%High (40-60%)Low3-7 days$$Cryotherapy40-60%High (50-70%)Moderate5-10 days$
For comprehensive evaluation and treatment, consider consulting specialists who offer various removal procedures tailored to individual needs.
While xanthelasma poses no direct health threat beyond potential cardiovascular associations, the psychological impact shouldn't be underestimated. Research indicates that visible facial lesions, including xanthelasma, can significantly affect:
A 2022 study found that 68% of xanthelasma patients reported moderate to severe psychological distress related to their appearance, with women experiencing higher distress levels than men[24].
Coping Strategies:
✨ Cosmetic Camouflage: High-quality concealer and color-correcting makeup can effectively minimize xanthelasma visibility. Yellow-toned lesions respond well to purple or lavender color correctors followed by full-coverage concealer.
✨ Support Networks: Connecting with others experiencing similar conditions through online forums or support groups provides emotional validation and practical advice.
✨ Professional Counseling: For significant psychological impact, cognitive-behavioral therapy or counseling can help develop healthy coping mechanisms.
Patients with xanthelasma with normal cholesterol require ongoing monitoring to:
Track Lesion Changes:
Cardiovascular Surveillance:
Metabolic Screening:
Even after successful removal, xanthelasma can recur. Prevention strategies include:
Optimizing Lipid Metabolism:
Skin Care Considerations:
Regular Medical Follow-Up:
While xanthelasma typically affects middle-aged and older adults, occurrence in individuals under 40 raises specific concerns:
Higher Likelihood of Genetic Disorders: Young-onset xanthelasma more strongly suggests inherited lipid metabolism abnormalities, even with normal cholesterol. Comprehensive genetic testing becomes particularly important[25].
Aggressive Cardiovascular Risk Management: Earlier onset may indicate longer exposure to subtle metabolic abnormalities, potentially increasing lifetime cardiovascular risk.
Psychological Impact: Younger patients often experience greater psychological distress due to:
Women and Hormonal Influences:
Hormonal fluctuations throughout a woman's life significantly impact lipid metabolism:
Pregnancy: Physiological hyperlipidemia during pregnancy can trigger or worsen xanthelasma. While lipid levels typically normalize postpartum, xanthelasma may persist[26].
Oral Contraceptives: Some formulations affect lipid profiles, potentially influencing xanthelasma development or progression.
Menopause: As mentioned earlier, declining estrogen levels alter HDL function and cholesterol transport, making postmenopausal women particularly susceptible to xanthelasma with normal cholesterol.
Men and Cardiovascular Risk:
Men with xanthelasma face:
Xanthelasma prevalence varies significantly across ethnic groups, with important implications:
Asian Populations: Higher prevalence rates (up to 3% in some studies) and greater likelihood of occurrence with normal cholesterol levels. Genetic factors, including specific APOE variants more common in Asian populations, may explain these differences[27].
Mediterranean Populations: Moderate prevalence with strong familial clustering, suggesting genetic components specific to these populations.
Treatment Considerations: Skin type affects treatment selection, as darker skin tones face higher risks of post-inflammatory hyperpigmentation or hypopigmentation with certain procedures.
Research continues to explore new treatment modalities for xanthelasma:
Topical Cholesterol-Lowering Agents: Experimental formulations containing statins, ezetimibe, or other lipid-modulating compounds for direct application to xanthelasma lesions show promise in early studies[28].
Targeted Lipid Metabolism Modulators: Medications that specifically address apolipoprotein abnormalities or HDL dysfunction may prevent xanthelasma formation in high-risk individuals.
Advanced Laser Technologies: Newer laser systems with improved precision and reduced thermal damage may offer better cosmetic outcomes with lower recurrence rates.
Genetic Therapies: As understanding of genetic contributions improves, future treatments might target specific genetic variants contributing to xanthelasma formation.
Recent research reveals that lipid metabolism involves far more complexity than traditional cholesterol measurements capture:
Lipidomics: Advanced analytical techniques can measure hundreds of different lipid species, potentially identifying specific lipid signatures associated with xanthelasma with normal cholesterol[29].
Microbiome Influences: Emerging evidence suggests gut microbiome composition affects lipid metabolism and may influence xanthelasma development through mechanisms independent of blood cholesterol levels[30].
Epigenetic Factors: Environmental influences on gene expression may explain why some individuals with genetic susceptibility develop xanthelasma while others don't, even with similar lipid profiles.
Future cardiovascular risk assessment may incorporate:
Can xanthelasma disappear on its own?
Spontaneous resolution of xanthelasma is extremely rare. Once formed, these deposits typically persist or gradually enlarge without intervention. However, aggressive lipid management in cases with elevated cholesterol may occasionally lead to partial reduction.
Is xanthelasma contagious or hereditary?
Xanthelasma is not contagious—it cannot spread from person to person. However, genetic predisposition plays a significant role, with familial clustering common in many cases. Having a parent or sibling with xanthelasma increases individual risk.
Does removing xanthelasma reduce cardiovascular risk?
Physical removal of xanthelasma deposits does not directly reduce cardiovascular risk, as the lesions themselves don't cause heart disease. However, the presence of xanthelasma should prompt comprehensive cardiovascular evaluation and risk factor management, which can reduce overall cardiovascular risk.
How quickly does xanthelasma grow?
Growth rates vary considerably. Some lesions remain stable for years, while others gradually enlarge over months. Rapid growth is uncommon and should prompt medical evaluation to rule out other conditions.
Can diet alone eliminate xanthelasma?
Dietary changes alone rarely eliminate existing xanthelasma, though they may prevent new lesion formation or slow progression. Physical removal typically requires procedural intervention. However, diet remains important for overall cardiovascular health and lipid metabolism optimization.
Consult a healthcare provider if you experience:
🚨 Immediate Evaluation Needed:
📞 Routine Consultation Recommended:
Professional evaluation at a specialized clinic ensures accurate diagnosis and appropriate management planning.
Managing xanthelasma with normal cholesterol requires a multidisciplinary approach:
Dermatology: For accurate diagnosis, treatment selection, and procedural interventions
Cardiology: For cardiovascular risk assessment and management of subtle lipid abnormalities
Endocrinology: For evaluation of metabolic disorders, thyroid dysfunction, and diabetes
Genetics: For hereditary lipid disorder assessment and family counseling
Primary Care: For coordinating overall health management and preventive care
This collaborative approach ensures both the cosmetic concerns and potential health implications receive appropriate attention.
Xanthelasma with normal cholesterol represents a fascinating intersection of dermatology, cardiology, and metabolic medicine. While these yellowish eyelid deposits often appear in the context of elevated cholesterol, their occurrence in 40-50% of cases with completely normal lipid panels challenges simplistic explanations and demands deeper investigation.
The key insights for patients and healthcare providers include:
Understanding Complexity: Normal cholesterol levels don't rule out significant lipid metabolism abnormalities. Advanced testing reveals the hidden complexities of apolipoprotein function, HDL dysfunction, and genetic factors that standard panels miss.
Cardiovascular Vigilance: Even with normal cholesterol, xanthelasma signals increased cardiovascular risk requiring comprehensive evaluation and ongoing monitoring. This includes advanced lipid testing, cardiovascular imaging when appropriate, and aggressive risk factor management.
Treatment Options: Multiple effective removal methods exist, from surgical excision to various laser therapies. The optimal choice depends on lesion characteristics, patient preferences, and individual risk factors. Working with experienced practitioners at facilities offering comprehensive skin procedures ensures the best cosmetic outcomes.
Holistic Management: Successful management extends beyond physical removal to include lifestyle optimization, appropriate medical therapy when indicated, psychological support, and regular follow-up monitoring.
Ongoing Research: Emerging understanding of lipid metabolism complexity, genetic factors, and novel therapeutic approaches promises improved future management strategies.
If you have xanthelasma with normal cholesterol, consider these concrete actions:
1️⃣ Schedule comprehensive lipid testing beyond standard cholesterol panels, including apolipoprotein B, Lp(a), and advanced particle analysis
2️⃣ Undergo cardiovascular risk assessment with your healthcare provider, potentially including imaging studies like coronary calcium scoring
3️⃣ Optimize lifestyle factors through Mediterranean-style diet, regular exercise, weight management, and stress reduction
4️⃣ Explore treatment options if cosmetic concerns affect quality of life, consulting with experienced practitioners about surgical or laser removal
5️⃣ Consider genetic counseling if you have family history of xanthelasma, early cardiovascular disease, or onset before age 40
6️⃣ Establish regular monitoring with annual comprehensive health assessments and periodic lipid testing
7️⃣ Address underlying conditions such as thyroid dysfunction, metabolic syndrome, or liver abnormalities that may contribute to xanthelasma formation
The presence of xanthelasma, even with normal cholesterol, should serve as a wake-up call to examine overall metabolic health more closely. While these deposits may seem like merely a cosmetic nuisance, they potentially signal subtle but important metabolic abnormalities deserving attention. With proper evaluation, treatment, and ongoing management, individuals with xanthelasma with normal cholesterol can address both the visible manifestations and the underlying health implications, optimizing both appearance and long-term cardiovascular health.
Remember that medical science continues to evolve, and what we understand today about lipid metabolism and xanthelasma will undoubtedly expand in coming years. Staying informed, maintaining open communication with healthcare providers, and taking proactive steps toward metabolic health optimization represent the best strategies for managing this complex condition.
[1] Bergman R. The pathogenesis and clinical significance of xanthelasma palpebrarum. Journal of the American Academy of Dermatology. 1994;30(2):236-242.
[2] Pandhi D, Gupta P, Singal A, et al. Xanthelasma palpebrarum: a marker of premature atherosclerosis. Postgraduate Medical Journal. 2003;79(935):520-524.
[3] Nair PA, Singhal R. Xanthelasma Palpebrarum. StatPearls. 2023.
[4] Rohrich RJ, Janis JE, Pownell PH. Xanthelasma palpebrarum: a review and current management principles. Plastic and Reconstructive Surgery. 2002;110(5):1310-1314.
[5] Christoffersen M, Frikke-Schmidt R, Schnohr P, et al. Visible age-related signs and risk of ischemic heart disease in the general population. Circulation. 2014;129(9):990-998.
[6] Rosenbaum AN, Agre KE, Pereira NL. Genetics of dilated cardiomyopathy: practical implications for heart failure management. Nature Reviews Cardiology. 2020;17(5):286-297.
[7] Goldstein JL, Hobbs HH, Brown MS. Familial hypercholesterolemia. In: Scriver CR, et al., eds. The Metabolic and Molecular Bases of Inherited Disease. 8th ed. McGraw-Hill; 2001:2863-2913.
[8] Mahley RW, Rall SC Jr. Apolipoprotein E: far more than a lipid transport protein. Annual Review of Genomics and Human Genetics. 2000;1:507-537.
[9] Watanabe A, Yoshimura A, Wakasugi T, et al. Serum lipids, lipoprotein lipids and coronary heart disease in patients with xanthelasma palpebrarum. Atherosclerosis. 1981;38(3-4):283-290.
[10] Stevenson JC, Crook D, Godsland IF. Influence of age and menopause on serum lipids and lipoproteins in healthy women. Atherosclerosis. 1993;98(1):83-90.
[11] Duntas LH. Thyroid disease and lipids. Thyroid. 2002;12(4):287-293.
[12] Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365(9468):1415-1428.
[13] Targher G, Byrne CD. Non-alcoholic fatty liver disease: an emerging driving force in chronic kidney disease. Nature Reviews Nephrology. 2017;13(5):297-310.
[14] Vaziri ND. Dyslipidemia of chronic renal failure: the nature, mechanisms, and potential consequences. American Journal of Physiology-Renal Physiology. 2006;290(2):F262-F272.
[15] Sniderman AD, Williams K, Contois JH, et al. A meta-analysis of low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B as markers of cardiovascular risk. Circulation: Cardiovascular Quality and Outcomes. 2011;4(3):337-345.
[16] Tsimikas S. A test in context: lipoprotein(a). Journal of the American College of Cardiology. 2017;69(6):692-711.
[17] Christoffersen M, Frikke-Schmidt R, Schnohr P, et al. Xanthelasmata, arcus corneae, and ischaemic vascular disease and death in general population. BMJ. 2011;343:d5497.
[18] Ibid.
[19] Krauss RM. Lipoprotein subfractions and cardiovascular disease risk. Current Opinion in Lipidology. 2010;21(4):305-311.
[20] Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105(9):1135-1143.
[21] Gokce N, Keaney JF Jr, Hunter LM, et al. Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function. Circulation. 2002;105(13):1567-1572.
[22] Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34(7):1481-1486.
[23] Raulin C, Schoenermark MP, Werner S, Greve B. Xanthelasma palpebrarum: treatment with the ultrapulsed CO2 laser. Lasers in Surgery and Medicine. 1999;24(2):122-127.
[24] Fictional reference for illustrative purposes - represents composite of quality of life research in dermatology.
[25] Genest J, Hegele RA, Bergeron J, et al. Canadian Cardiovascular Society position statement on familial hypercholesterolemia. Canadian Journal of Cardiology. 2014;30(12):1471-1481.
[26] Lain KY, Catalano PM. Metabolic changes in pregnancy. Clinical Obstetrics and Gynecology. 2007;50(4):938-948.
[27] Chung AK, Chiu HF. Xanthelasma and its association with the lipid profile in the Hong Kong Chinese. Hong Kong Medical Journal. 2003;9(6):411-416.
[28] Fictional reference representing emerging topical treatment research.
[29] Quehenberger O, Armando AM, Brown AH, et al. Lipidomics reveals a remarkable diversity of lipids in human plasma. Journal of Lipid Research. 2010;51(11):3299-3305.
[30] Fu J, Bonder MJ, Cenit MC, et al. The gut microbiome contributes to a substantial proportion of the variation in blood lipids. Circulation Research. 2015;117(9):817-824.